
Every time you take an antibiotic when you don’t need it, you’re not just helping yourself-you’re helping bacteria become stronger. It sounds backward, but it’s true. Antibiotic resistance isn’t science fiction. It’s happening right now, in hospitals, farms, and even your own kitchen sink. And it’s getting worse.
How Bacteria Outsmart Antibiotics
Antibiotics don’t kill bacteria because they’re powerful poisons. They target specific weak spots-like the tools bacteria use to build their cell walls or copy their DNA. But bacteria aren’t passive. They evolve. Fast.
When exposed to antibiotics, some bacteria survive because they’ve got mutations in their genes. These aren’t random accidents. They’re survival tricks passed down. One common mutation happens in the gyrA gene, which changes how bacteria respond to drugs like ciprofloxacin. Another targets the ampC gene, making them resistant to amoxicillin. In some cases, bacteria develop mutations in efflux pumps-tiny molecular vacuums that spit antibiotics back out before they can do damage.
What’s scary is how quickly this happens. In lab studies, bacteria exposed to low doses of antibiotics developed high-level resistance in as few as 150 generations. That’s not years. That’s weeks. And once resistance stabilizes, it sticks. Mutations that once helped them survive become permanent fixes.
It’s not just one gene. It’s a whole system. Some bacteria switch on genes that alter their metabolism-shifting energy use to survive drug stress. Others use epigenetic changes, like DNA methylation, to temporarily shut down vulnerable pathways. These aren’t permanent, but they buy time. And in that time, permanent mutations creep in.
It’s Not Just Antibiotics
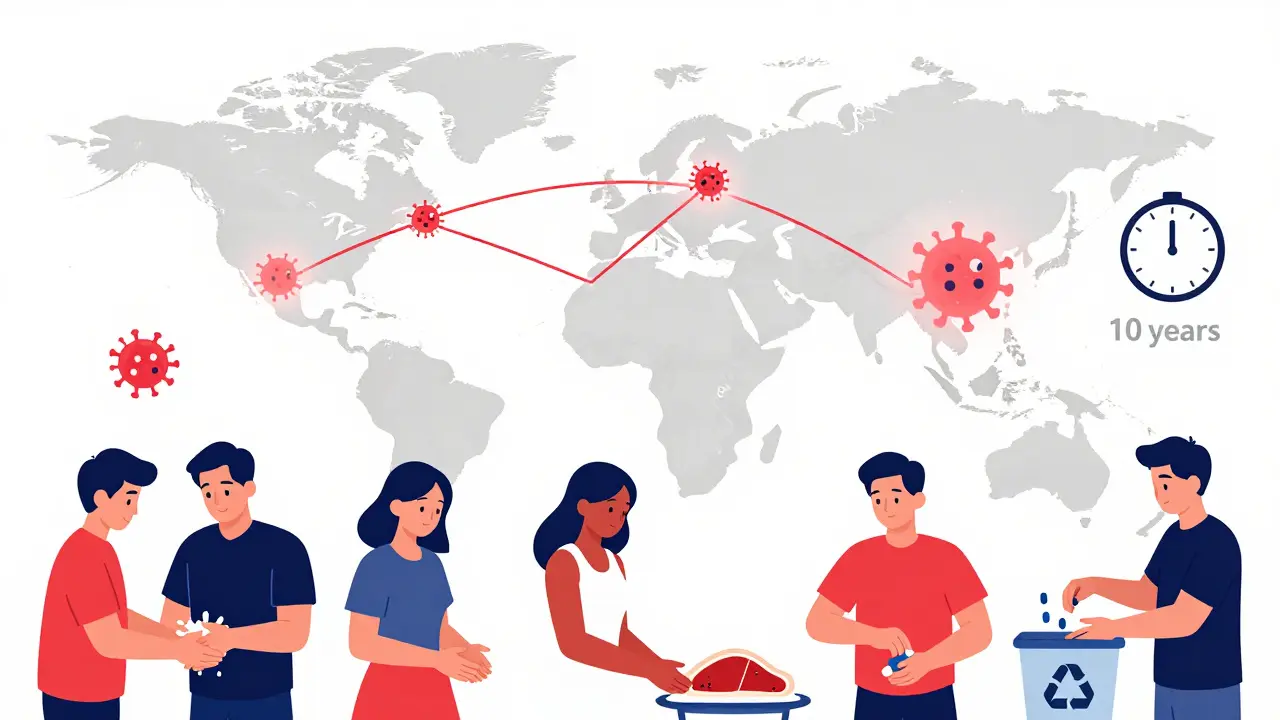
You might think resistance only comes from taking too many pills. But that’s only part of the story. Antibiotics are used heavily in farming-over 70% of all antibiotics sold in the U.S. go to livestock. These drugs aren’t always treating sick animals. Often, they’re given daily to keep them alive in crowded, unsanitary conditions. That’s a breeding ground for resistant bacteria.
And it doesn’t stop there. New research shows that common non-antibiotic drugs-like painkillers, antidepressants, and even antacids-can make it easier for bacteria to grab resistance genes from their neighbors. This isn’t just about your medicine cabinet. It’s about what ends up in water systems, soil, and food chains.
One study found that when bacteria were exposed to low levels of tetracycline, they didn’t just mutate. They rewired their own gene control systems. A tiny insertion in their DNA-called an IS2 transposon-switched on a pump gene that normally doesn’t even handle tetracycline. Suddenly, they could pump it out. That’s not evolution by accident. That’s evolution by opportunity.
Why You’re Part of the Problem (And the Solution)
The CDC says nearly one in three antibiotic prescriptions in U.S. clinics are unnecessary. That’s 47 million courses a year given for colds, flu, or sore throats-things caused by viruses, not bacteria. Antibiotics do nothing for viruses. But people still ask for them. Doctors still give them. And bacteria keep winning.
In the EU, antibiotic resistance causes over 33,000 deaths every year. The economic cost? Over €1.5 billion. In low-income countries, the problem is even worse. Many don’t have access to proper diagnostics. So doctors guess. They prescribe. And resistance spreads.
But here’s the good news: you can stop it. Not by taking more drugs. By taking fewer.
- If your doctor says you don’t need antibiotics, trust them. Viral infections get better with rest, fluids, and time.
- Never pressure a doctor for antibiotics. They’re not a quick fix.
- Never save leftover antibiotics for next time. Bacteria love leftovers.
- Never share antibiotics. What works for one person might be useless-or dangerous-for another.
- If you’re prescribed antibiotics, finish the full course. Stopping early leaves the toughest bacteria alive.

The Bigger Picture: One Health
Antibiotic resistance doesn’t care about borders. It moves between people, animals, and the environment. That’s why experts talk about One Health-the idea that human, animal, and environmental health are linked.
Resistant bacteria from farm animals can end up on your veggies. Water runoff from hospitals carries drug-resistant genes into rivers. Travelers bring resistant strains across continents. You can’t fix this by just changing your own habits. You need systems to change too.
That’s why countries like New Zealand, Canada, and the Netherlands have strict rules on antibiotic use in farming. That’s why hospitals now have antimicrobial stewardship teams-doctors and pharmacists who review every antibiotic prescription to make sure it’s truly needed. These programs cut unnecessary use by 20-30% without hurting patient outcomes.
But progress is slow. Only 17 of the 67 new antibiotics in development today target the most dangerous superbugs. And only three are truly new-designed to bypass existing resistance. Most are tweaks of old drugs. That’s like trying to stop a flood with a bucket.
What’s Being Done? And What’s Missing?
Scientists are trying new tricks. CRISPR gene editing is being tested to cut resistance genes out of bacteria. Metabolomics helps spot how bacteria change their energy use to survive drugs. Bioinformatics tools predict which mutations are likely to appear next.
The FDA just approved new testing guidelines for cefiderocol, a last-resort antibiotic for carbapenem-resistant infections. That’s progress. But testing is still too slow. Many hospitals still rely on old methods that take days. By then, the infection has spread.
And while 150 countries have national plans to fight resistance, only 35% of low-income nations have fully implemented theirs. High-income countries are at 75%. That gap is deadly.
What’s missing? Investment. Coordination. Accountability. We know how to slow resistance. We just haven’t made it a priority.
What You Can Do Today
You don’t need a lab coat to fight antibiotic resistance. You just need to be smart.
- Ask your doctor: “Is this really an infection that needs antibiotics?”
- Wash your hands. It’s the oldest, most effective way to stop the spread of resistant bacteria.
- Choose meat from farms that don’t use antibiotics routinely. Look for labels like “raised without antibiotics.”
- Don’t flush old meds down the toilet. Take them to a pharmacy drop-off.
- Support policies that fund better diagnostics and restrict antibiotic use in agriculture.
Every time you say no to an unnecessary antibiotic, you’re not just protecting yourself. You’re protecting the next generation. Because if we keep going like this, we’ll reach a point where common infections-like a scratched knee or a urinary tract infection-become deadly again.
We’ve already seen the warning signs. Now we need to act.
Can antibiotic resistance be reversed?
In some cases, yes-but it’s slow. If antibiotic use drops significantly, resistant bacteria may lose their advantage and become less common over time. But the genes don’t disappear. They stay hidden in bacterial populations, ready to resurface if antibiotics are used again. Stopping misuse is the only way to prevent them from taking over.
Are natural remedies effective against resistant bacteria?
Some natural substances like honey, garlic, or tea tree oil show antimicrobial effects in lab tests. But they’re not replacements for antibiotics in serious infections. They lack the precision, dosage control, and proven safety needed for clinical use. Relying on them instead of medical care can delay treatment and make infections worse.
Why do doctors still prescribe antibiotics when they’re not needed?
Pressure from patients, time constraints, and uncertainty about diagnosis play a role. Many patients expect a prescription. Doctors may give one to avoid conflict-even if they know it’s not necessary. Better education for both doctors and patients is key to changing this.
Can I get antibiotic-resistant infections from my pets?
Yes. Pets can carry resistant bacteria, especially if they’ve been treated with antibiotics. You can pick them up through direct contact, handling pet waste, or even through contaminated surfaces. Wash your hands after touching pets, especially if they’re on antibiotics.
Is there a new antibiotic coming soon that will solve this?
There are new drugs in development, but they’re not magic bullets. Most are modifications of existing ones. Only a few are truly novel. And bacteria will eventually adapt to them too. The real solution isn’t more drugs-it’s using the ones we have wisely.
How long does it take for resistance to develop after taking antibiotics?
Resistance can develop in days-even within a single treatment. The bacteria already in your body may have mutations that let them survive. Taking antibiotics kills off the weak ones, leaving the strong ones to multiply. That’s why finishing the full course matters-it helps wipe out the survivors.
Does using antibiotics for acne contribute to resistance?
Yes. Long-term, low-dose antibiotics for acne are a major driver of resistance. Studies show people on these regimens carry more resistant bacteria in their skin and gut. Dermatologists now recommend shorter courses and combining antibiotics with topical treatments to reduce risk.
Can I test myself for antibiotic-resistant bacteria?
Not easily. Testing requires a lab culture and specialized equipment. Most people won’t know they’re carrying resistant bacteria unless they get a serious infection and the first antibiotic doesn’t work. That’s why prevention-avoiding unnecessary use-is so important.
What Comes Next?
We’re not running out of antibiotics. We’re running out of time to use them wisely. The next decade will determine whether we stay ahead of superbugs-or lose the battle for good.
It’s not about fear. It’s about responsibility. Every pill you don’t take, every farm you support that doesn’t overuse drugs, every conversation you have about this issue-it adds up.
Antibiotics saved millions. But they’re not invincible. They need us to protect them.
13 Comments
suhani mathur
December 25, 2025 AT 00:06 AM
oh wow so antibiotics are bad now? next you’ll tell me oxygen causes cancer because some people get pneumonia. chill. your post is 90% accurate but the tone is like you’re handing out blame like it’s halloween candy. some of us are just trying not to die of a UTI. thanks for the info though. 🙃
Diana Alime
December 25, 2025 AT 20:17 PM
okay but like... why am i supposed to care? i got a sinus infection last winter and the doc gave me azithromycin and i felt better. that’s it. i dont have time to read 17 studies on efflux pumps. also i’m pretty sure my chicken nuggets have more antibiotics than protein. who’s gonna fix that? not me. i have a dog and a toddler and zero energy. 🤷♀️
Adarsh Dubey
December 27, 2025 AT 01:09 AM
the science here is solid. resistance evolves fast because bacteria reproduce faster than we can update our medical protocols. what’s interesting is how epigenetic changes act as a temporary shield while permanent mutations develop. it’s not just about misuse-it’s about systemic failure in diagnostics, agriculture, and global equity. we need better tools, not just better will.
Jeffrey Frye
December 28, 2025 AT 09:20 AM
you say "you’re helping bacteria become stronger" like it’s a moral failing. but what if i’m just tired of waiting three days for a cold to go away? what if my job doesn’t give me sick days? what if my kid’s fever hits 103 and the ER says "wait it out"? this isn’t about laziness. it’s about broken systems. and now you want me to feel guilty for surviving?
Chris Buchanan
December 28, 2025 AT 23:39 PM
you’re right. and here’s the kicker: every time you finish your antibiotics, you’re doing a tiny favor for humanity. seriously. it’s like doing the dishes. nobody notices, but if everyone skips it, the whole kitchen drowns. you don’t have to be a hero. just be consistent. 💪
Wilton Holliday
December 30, 2025 AT 16:22 PM
you know what’s wild? bacteria have been doing this for 2 billion years. we’re just the latest species to try and control them. 🤯 maybe instead of fighting them, we should learn from them. they adapt. they cooperate. they survive. we need to be more like bacteria. and less like people who hoard antibiotics like toilet paper in 2020 😅
Joseph Manuel
December 31, 2025 AT 06:29 AM
the assertion that antibiotic resistance is primarily driven by individual behavior is empirically unsound. the literature indicates that agricultural use accounts for approximately 70% of global consumption. the focus on patient compliance is a classic case of moralization masking structural neglect. the onus must be placed on regulatory agencies and agribusiness, not the layperson.
Harsh Khandelwal
January 1, 2026 AT 14:56 PM
so let me get this straight-big pharma poisons the water, farms pump antibiotics into cows like soda, and now they want us to feel bad for popping a pill? nah. they’re selling us the cure while selling the disease. next they’ll tell us to buy the ‘super-antibiotic’ that costs $5000 and comes with a 12-page warning label. i’m not buying. i’m not playing. i’m just gonna eat garlic and pray.
Andy Grace
January 3, 2026 AT 11:35 AM
i live in australia and we’ve got strict rules on farm antibiotics. it’s not perfect, but it’s better than nothing. i’ve seen farmers switch to probiotics and better hygiene-and their animals are healthier. change is possible. it’s just slow. and quiet. and doesn’t make headlines. but it’s happening.
Delilah Rose
January 3, 2026 AT 18:51 PM
i think what’s missing from this conversation is the emotional weight of this issue. it’s not just about science or policy-it’s about parents who watch their kids get sicker because the first antibiotic didn’t work, or elderly people who end up in the hospital for a simple cut because nothing works anymore. we’re not just talking about bacteria-we’re talking about the quiet erosion of trust in modern medicine. and that’s terrifying. we need to talk about this like it’s a family emergency, because it is.
Spencer Garcia
January 4, 2026 AT 11:27 AM
finish the course. don’t save leftovers. ask questions. wash hands. that’s it. you don’t need a PhD to help.
Abby Polhill
January 4, 2026 AT 17:12 PM
the real bottleneck isn’t the drugs-it’s the diagnostic gap. we’re still using culture-based methods from the 1950s while bacteria are running on CRISPR-enhanced turbo mode. we need point-of-care genomic screening. decentralized, rapid, affordable. until then, we’re fighting a war with slide rules. the tech exists. the funding doesn’t.




siddharth tiwari
December 24, 2025 AT 07:02 AM
theyve been putting fluoride in the water since the 50s to weaken our immune systems so we need more antibiotics lol. its all a pharma psyop. i saw a video on telegram where a guy in canada proved bacteria turn into robots when you take amoxicillin. theyre already in our food. dont trust the FDA. they work for big pharma.