When you pick up a prescription and see a different name on the bottle - maybe it’s no longer Advil but ibuprofen - you might wonder: Is this the same thing? Will it work just as well? Could it hurt me? These aren’t just casual questions. They’re life-or-death concerns for millions of people who rely on medications every day. The answer lies in something called bioequivalence - a scientific process that’s quietly protecting your health every time you take a generic drug.
What Bioequivalence Really Means
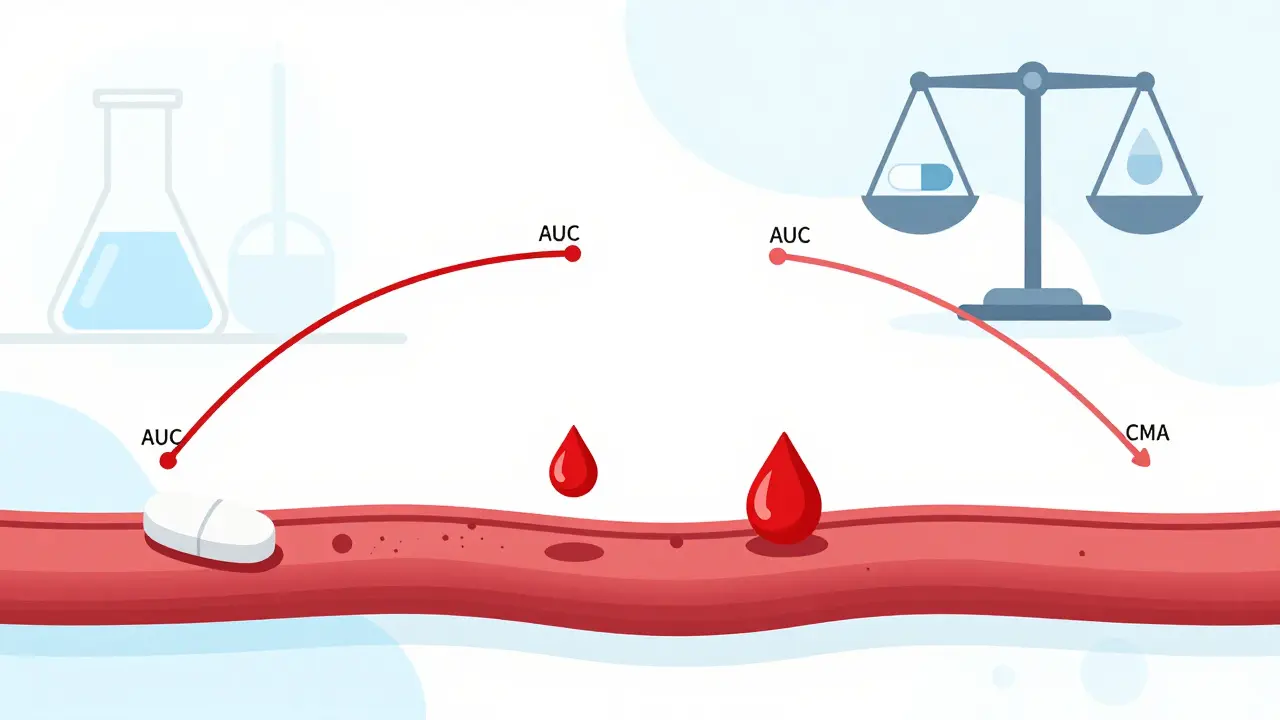
Bioequivalence isn’t marketing jargon. It’s a precise, measurable standard. It means that a generic drug delivers the same amount of active ingredient into your bloodstream at the same speed as the brand-name version. Not close. Not almost. The same. The test checks two things: how much of the drug gets into your blood (called AUC), and how fast it gets there (called Cmax). For most drugs, regulators require that the generic’s values fall between 80% and 125% of the brand’s. That’s not a wide gap - it’s a tight window designed to ensure no meaningful difference in how the drug works in your body.This standard wasn’t invented overnight. It came from the 1984 Hatch-Waxman Act in the U.S., which created the modern pathway for generic drugs. Before that, patients had few affordable options. Now, 90% of prescriptions filled in the U.S. are generics - but they make up only 23% of total drug spending. In 2020 alone, generics saved the American healthcare system $313 billion. That’s money kept in people’s pockets and in hospital budgets. But none of that matters if the drugs aren’t safe.
How Testing Keeps You Safe
Bioequivalence studies don’t happen in a lab with a single vial. They’re clinical trials - usually done in healthy volunteers. Participants take the brand-name drug one day, then the generic another day, after a washout period. Blood samples are drawn over hours, sometimes days. The data is analyzed using strict statistical methods. The goal? To prove that the two drugs behave identically in the body.It’s not just about the main ingredient. Some drugs break down into active metabolites - chemicals that also do the work. For example, the blood pressure drug losartan turns into EXP-3174, which is actually more powerful. So testing doesn’t stop at losartan. Scientists must measure both. If the generic doesn’t produce the same level of that metabolite, it’s not bioequivalent - and it gets rejected.
For drugs with a narrow therapeutic index - like warfarin, levothyroxine, or phenytoin - the stakes are even higher. A tiny difference can mean a clot, a seizure, or thyroid failure. That’s why regulators tighten the bioequivalence range to 90-111% for these drugs. After the FDA updated its guidance for levothyroxine in 2012, manufacturers had to meet stricter standards. Patient reviews on Drugs.com show that 58% now say the generic works the same as the brand. That’s not luck. That’s science.
Why Some Patients Worry - and Why They’re Usually Wrong
You’ve probably heard stories. Someone switched from brand to generic antidepressant and felt “off.” Another person switched to generic seizure medication and had a breakthrough seizure. These are real experiences. But they’re rarely caused by bioequivalence failure.The FDA tracks every adverse event reported through its FAERS system. Between 2020 and 2023, only 0.07% of all drug-related adverse events involved generics that had passed bioequivalence testing. Meanwhile, 2.3% involved brand-name drugs. That’s not a typo. It means generics are statistically safer in reported harm - not because they’re better, but because they’re more consistent.
Why the disconnect? A lot of it comes down to expectation bias. If you’ve been on a brand-name drug for years, your brain associates that pill with feeling stable. When the shape, color, or name changes, your body might react to the psychological shift, not the chemistry. Pharmacists on Reddit’s r/pharmacy often point out that when patients report problems after switching, the same issue often appears with different generics - meaning the problem isn’t the drug, it’s the switch itself.
Still, there are exceptions. Some people are more sensitive. That’s why doctors and pharmacists still monitor patients closely after a switch, especially with high-risk medications. But that’s not because bioequivalence fails. It’s because medicine is personal. And science gives us the tools to manage that.
The Global Picture - Not All Rules Are the Same
Bioequivalence isn’t just an American standard. It’s global. The European Medicines Agency, Health Canada, Australia’s TGA, New Zealand’s Medsafe, and the World Health Organization all require it. But the details vary. Japan insists on fasting studies even if the brand is meant to be taken with food. The U.S. usually tests both fasting and fed states. Brazil requires a minimum set of medical checks no matter what the study design is.These differences make it harder for generic manufacturers to sell worldwide. One formulation that passes in the U.S. might fail in Japan because of a single dietary rule. That’s why groups like the International Pharmaceutical Regulators Programme are pushing for harmonization. They want one set of rules - not 134 different ones (that’s how many countries now require bioequivalence testing).
And it’s getting more complex. Drugs like inhalers, creams, and eye drops don’t enter the bloodstream the same way pills do. Their effectiveness depends on where they land in the body - the lung, the skin, the eye. For these, traditional blood tests don’t work. Regulators are now using new methods: in-vitro tests that mimic skin absorption, imaging to track drug delivery, and even computer modeling to predict how the drug behaves. The FDA accepted 17 such computer-based submissions in 2022 - up from just 3 in 2018. That’s progress.
Who Pays for This - And Why It Matters
Running a bioequivalence study isn’t cheap. It costs between $1 million and $2 million per drug. It takes 12 to 18 months. You need specialized labs, trained staff, and volunteers who follow strict diets and schedules. Contract research organizations like PPD and WuXi AppTec make billions from this work. But here’s the flip side: without this testing, there’d be no affordable generics.Imagine if every drug company had to run full clinical trials for every generic - like they do for new drugs. The cost would be astronomical. Prices would stay high. Millions of people would go without treatment. Bioequivalence testing is the bridge between innovation and access. It lets generics enter the market without repeating every expensive trial. It’s efficient. It’s ethical. And it’s backed by decades of data.

The Future of Safe Medications
The next frontier? Using artificial intelligence to predict bioequivalence. Instead of running human trials for every new generic, scientists are training AI to analyze dissolution profiles - how quickly a pill breaks down in fluid - and predict whether it will behave like the brand. The FDA’s 2023-2027 plan calls this a priority. If it works, it could cut development time and cost even further, without sacrificing safety.Meanwhile, patient advocacy groups like AARP are cheering. They point out that generics saved Medicare Part D beneficiaries $1.7 trillion from 2006 to 2020. That’s not just a number. That’s insulin for diabetics. Blood pressure pills for seniors. Cholesterol meds for people who can’t afford the brand.
As generic use climbs to 94% of U.S. prescriptions by 2027, the need for strong bioequivalence standards won’t fade - it’ll grow. Because the more people who depend on these drugs, the more we can’t afford to cut corners.
What You Can Do
If you’re prescribed a generic drug, don’t panic. The system is built to protect you. But stay informed. Talk to your pharmacist. Ask: “Has this been tested to work the same as the brand?” If you notice a change in how you feel after switching - even if it’s subtle - tell your doctor. Don’t assume it’s “all in your head.”And if you’re ever told a generic isn’t available, ask why. Sometimes it’s just a pharmacy stock issue. Other times, it’s because the drug has a narrow therapeutic index and the pharmacy wants to avoid risk. That’s not always bad - it’s just a reminder that bioequivalence isn’t one-size-fits-all. It’s tailored. And that’s why it works.
Are generic drugs as safe as brand-name drugs?
Yes. Generic drugs must pass strict bioequivalence testing to prove they deliver the same amount of active ingredient at the same rate as the brand-name version. Regulatory agencies like the FDA and EMA require this before approval. Once approved, generics are considered therapeutically equivalent. Adverse event data shows that generics are not more dangerous - and often less frequently linked to reported side effects than brand-name drugs.
Why do some people say generics don’t work for them?
Many reports of problems after switching to generics are due to psychological factors, changes in pill appearance, or coincidental health shifts - not actual differences in drug performance. While rare cases of sensitivity exist, especially with narrow therapeutic index drugs like warfarin or levothyroxine, these are monitored closely by doctors and regulators. Systematic failures due to bioequivalence issues are extremely rare and would trigger a recall.
Is bioequivalence testing the same for all types of drugs?
No. For most oral pills, bioequivalence is tested using blood samples from healthy volunteers. But for drugs like inhalers, creams, or eye drops, the drug doesn’t enter the bloodstream the same way. Regulators now use specialized methods - such as in-vitro tests, imaging, and computer modeling - to prove these products work the same. These are more complex and still evolving.
What’s the difference between generics and biosimilars?
Generics are chemically identical copies of small-molecule drugs. Biosimilars are copies of complex biological drugs made from living cells. Because biological drugs are harder to replicate exactly, biosimilars don’t need to prove bioequivalence in the same way. Instead, they undergo a full “totality of evidence” review - including structural analysis, immune response tests, and clinical trials - to prove they’re highly similar in safety and effectiveness.
Can I trust generics if I’m on a high-risk medication?
Yes - but with extra care. For drugs with a narrow therapeutic index (like warfarin, lithium, or levothyroxine), regulators use tighter bioequivalence limits (90-111%) and often require additional testing. Your doctor may monitor your blood levels more closely after switching. But the system is designed to protect you. If a generic fails to meet these stricter standards, it won’t be approved.




Wendy Lamb
February 3, 2026 AT 20:26 PM
Just wanted to say this is one of the clearest explanations of bioequivalence I’ve ever read. Seriously, if your doctor ever tells you generics are 'inferior,' hand them this. It’s not magic-it’s math, and it works.