When a drug warning pops up on your screen-maybe a black box alert or a safety notice from the FDA-it’s easy to assume it applies to every drug in that category. But that’s not always true. Some warnings hit every drug in a class. Others? Just one. Getting this wrong can mean avoiding a life-saving medication-or continuing one that’s risky. So how do you tell the difference between a class-wide safety alert and a drug-specific one?
What’s the Difference Between Class-Wide and Drug-Specific Alerts?
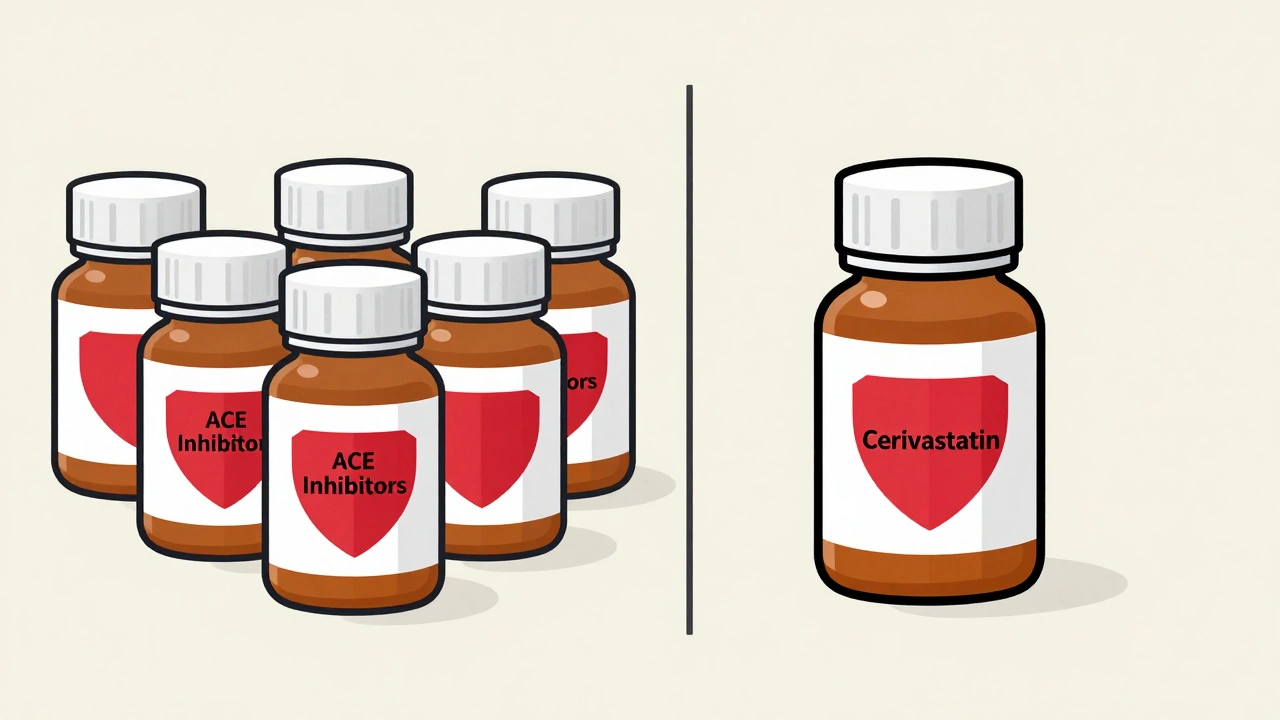
A class-wide safety alert means the risk applies to all drugs in a group because they work the same way. For example, all ACE inhibitors carry a risk of angioedema because they block the same enzyme. If one drug in the class causes it, the mechanism suggests others might too. That’s why, in 2023, the FDA updated labeling for all 12 testosterone products after studies showed they all raised blood pressure in some patients. A drug-specific alert, on the other hand, targets just one medication. Take cerivastatin. It was pulled from the market in 2001 because it caused dangerous muscle breakdown (rhabdomyolysis) at rates far higher than other statins. But rosuvastatin, atorvastatin, and simvastatin? They stayed on shelves. Why? Because their chemistry, metabolism, and dosing made them safer-even though they all lower cholesterol the same way. The confusion? It’s real. A 2022 survey of 1,200 U.S. doctors found 68% struggled to tell the difference. Primary care providers were even more unsure-73% admitted they sometimes guessed instead of checking.How the FDA Decides: The Evidence Behind the Warning
The FDA doesn’t slap on class-wide warnings lightly. They need more than one bad report. Here’s what they look for:- Multiple sources of data: At least three different drugs in the class showing the same problem in FAERS (the FDA’s adverse event database), which holds over 22 million reports.
- Statistical signals: A Proportional Reporting Ratio (PRR) above 2.0 and a Chi-squared value over 4.0 across multiple databases. This means the adverse event is happening way more often than random chance would explain.
- Biological plausibility: Does the drug’s mechanism explain the risk? If all drugs in the class block the same pathway, and one causes liver damage, others might too.
- Real-world consistency: Did multiple studies-clinical trials, registries, or insurance claims-show the same pattern?
- A single drug linked to 50+ cases of a rare side effect (like cerivastatin’s rhabdomyolysis cases).
- Unique metabolism: Some drugs are broken down by a liver enzyme that others aren’t, leading to toxic buildup.
- High-dose use: A drug might be safe at low doses but dangerous at high ones-only if it’s prescribed that way.
Why It Matters: Real-World Consequences
Mixing up these alerts can change how you treat patients-or yourself. In 2018, the FDA issued a class-wide warning for fluoroquinolone antibiotics (like ciprofloxacin and levofloxacin) due to disabling tendon and nerve damage. After that, overall use of the class dropped 17%. But that meant some patients with serious infections didn’t get the best treatment because doctors assumed all fluoroquinolones were too risky. Compare that to valdecoxib (Bextra), a COX-2 inhibitor pulled in 2004 due to heart attack risks. Celecoxib (Celebrex), another COX-2 inhibitor, stayed on the market. Why? Evidence showed celecoxib didn’t carry the same risk. That’s drug-specific. Prescribers could still use it safely-with monitoring. The problem? When warnings are inconsistent, trust erodes. In 2011, the FDA found that 33% of drug classes with black box warnings had them on some members but not others. One example: citalopram got a warning for QT prolongation (a heart rhythm issue) in 2011, but escitalopram-its nearly identical cousin-didn’t. Doctors were left guessing. Later research showed escitalopram carried the same risk. That delay cost time and safety.How to Check the Scope of a Warning Yourself
You don’t need to be a regulator to figure this out. Here’s how to verify:- Go to DailyMed (dailymed.nlm.nih.gov). Type in the drug name. Look at the “Warnings and Precautions” section. The FDA now labels alerts as either “Class Risk” or “Agent-Specific Risk” in the text.
- Check the FDA’s Drug Safety Communications. The archive (fda.gov/drugs/drug-safety-and-availability) filters by alert type. As of 2023, 18% were class-wide, 62% were drug-specific.
- Look at the label. If it says “All drugs in this class…” or “This risk has been observed across the class,” it’s class-wide. If it says “This agent has been associated with…” or “No similar reports with other drugs in this class,” it’s specific.
- Ask a pharmacist. Pharmacists are trained to catch these differences. Walgreens reported a 22% increase in verification time for class-wide alerts-because they have to check every alternative, not just one.
Common Mistakes and Misconceptions
People mix up three things all the time:- Drug recall classes (Class I, II, III) ≠ therapeutic classes. A Class I recall means the drug could cause death. That’s about severity, not scope. A Class I recall on one statin doesn’t mean all statins are dangerous.
- Similar names don’t mean similar risks. Cephalosporin antibiotics all end in “-cef,” but only a few (like ceftriaxone) carry high allergy risks. Don’t assume.
- One bad case doesn’t make a class warning. The FDA gets thousands of reports. They look for patterns, not outliers.

What’s Changing in Drug Safety Monitoring
The system is getting smarter. In 2023, the FDA launched a pilot using AI to predict class risks before they’re even noticed-by analyzing molecular structures and how drugs are metabolized. They’re also using data from 100 million patients through the National Evaluation System for health Technology (NEST) to spot trends faster. The 21st Century Cures Act (2016) now requires the FDA to evaluate entire classes when a signal emerges. That’s a big shift. Ten years ago, they might have just warned about one drug. Now, they ask: “Could this happen to others?” But challenges remain. A 2022 FDA-NIH report found that for 72% of drug classes, there’s not enough post-market data to be sure. That’s why some warnings are delayed-or wrong.What You Should Do
If you’re a patient: Don’t stop a medication because of a warning you don’t understand. Talk to your doctor or pharmacist. Ask: “Is this warning for all drugs like mine, or just this one?” If you’re a provider: Use the STEPS model-Safety, Tolerability, Effectiveness, Price, Simplicity. Ask: “Is there a safer, equally effective alternative?” Don’t let blanket warnings drive decisions. Use the data. If you’re in pharmacy or health IT: Push for systems that flag warning scope clearly. Many EHRs still show warnings without context. That’s dangerous.Final Thought
Drug safety isn’t about fear. It’s about precision. The goal isn’t to warn everyone about everything. It’s to warn the right people, about the right risks, at the right time. Class-wide alerts protect more people. Drug-specific alerts protect the integrity of the whole class. Getting it right saves lives-and keeps good drugs available.How do I know if a safety alert applies to all drugs in a class or just one?
Check the FDA’s Drug Safety Communications or DailyMed. The FDA now labels alerts as either "Class Risk" or "Agent-Specific Risk." If the warning mentions "all drugs in this class," "this mechanism," or "similar agents," it’s class-wide. If it names only one drug or says "this agent has been associated with," it’s specific. When in doubt, ask a pharmacist or check the full prescribing information.
Can a drug with a black box warning still be prescribed?
Yes. A black box warning doesn’t mean the drug is banned-it means the risk is serious enough to require special attention. Many drugs with black boxes, like insulin, warfarin, and certain antidepressants, are still first-line treatments. The warning tells you to monitor closely, use the lowest effective dose, and consider alternatives if the risk outweighs the benefit.
Why do some drugs in the same class have warnings and others don’t?
Because their chemistry, metabolism, dosing, or clinical use differs. For example, cerivastatin was withdrawn because it was metabolized in a way that caused dangerous muscle damage, while other statins weren’t. The FDA evaluates each drug individually, even within a class. Sometimes, evidence is strong for one drug but not others. This can lead to temporary inconsistencies, but the goal is always to match the warning to the actual risk.
Are class-wide warnings more common now than before?
Yes. Between 2000 and 2010, class-wide warnings made up 12% of FDA black box warnings. From 2011 to 2021, that rose to 18%. This is due to better data systems like FAERS, the 21st Century Cures Act requiring class evaluation, and AI tools that detect patterns across multiple drugs. Regulators are now more likely to ask, "Could this affect others?" before acting.
What should I do if I’m told to stop a medication because of a safety alert?
Don’t stop without talking to your provider. Ask: "Is this warning for this drug only, or all drugs like it?" If it’s drug-specific, there may be safe alternatives in the same class. If it’s class-wide, ask if there’s another class of medication that works just as well. Never make changes based on a headline or a vague warning-get the full context.
15 Comments
Conor Forde
December 2, 2025 AT 15:06 PM
YEAH RIGHT. ‘Class-wide’ is just Big Pharma’s way of covering their ass. They know 90% of docs won’t dig into DailyMed, so they slap ‘ALL DRUGS IN THIS CLASS’ on everything. Cerivastatin got axed? Cool. But why’s rosuvastatin still on the shelf? Same enzyme. Same class. Same damn mechanism. 🤷♂️ This isn’t science - it’s corporate damage control with a side of FDA laziness.
Declan O Reilly
December 4, 2025 AT 01:06 AM
It’s funny how we treat meds like they’re all the same. We say ‘statins’ like it’s one thing. But no - each one has its own metabolic fingerprint. Like how some are broken down by CYP3A4 and others by CYP2C9. One guy gets rhabdo because he’s a slow metabolizer. Another? Fine. It’s not about the class - it’s about the person. We need pharmacogenomics built into prescribing workflows. Not just warnings. Personalization. 🧬
Patrick Smyth
December 5, 2025 AT 21:47 PM
YOU ARE ALL IGNORANT. THE FDA IS A CORRUPT INSTITUTION. THEY LET DRUGS KILL PEOPLE ON PURPOSE TO PROTECT BIG PHARMA PROFITS. WHY DID THEY WAIT 10 YEARS TO WARN ABOUT ESCITALOPRAM’S QT PROLONGATION? BECAUSE THEY WERE PAID OFF. THIS ISN’T MEDICINE - IT’S A GAME. AND WE’RE THE Pawns.
Linda Migdal
December 7, 2025 AT 11:49 AM
Let me tell you something - if you’re not checking DailyMed before prescribing, you’re a liability. The FDA’s new ‘Class Risk’ / ‘Agent-Specific Risk’ tags are a game-changer. I’ve caught three misprescriptions in the last month because I read the label. America needs mandatory CME on this. No more guessing. No more ‘I thought it was the whole class.’
Tommy Walton
December 7, 2025 AT 16:37 PM
Bro. Think about it. 🤔 Class-wide alerts are like saying ‘all cars are dangerous because one had a faulty brake line.’ But what if that car had a 1970s brake system? 🚗💥 The rest are 2024 Teslas. We need nuance. Not blanket fear. Also, AI is gonna fix this. Soon. We’re entering the era of predictive pharmacovigilance. 🤖💊
James Steele
December 9, 2025 AT 16:27 PM
The real issue isn’t the classification - it’s the epistemic arrogance of regulatory bodies. They assume mechanistic similarity implies clinical equivalence. But pharmacokinetics is a labyrinth. One drug’s CYP2D6 polymorphism is another’s safety net. The FDA’s reliance on FAERS is archaic. We need longitudinal, real-world phenotyping - not just statistical signals. This isn’t medicine. It’s epidemiological guesswork with a government seal.
Louise Girvan
December 11, 2025 AT 07:12 AM
They’re hiding something. Always. Why did they only warn about citalopram and not escitalopram? Because the trials were rigged. The data was cherry-picked. And now? They’re using AI to ‘predict’ risks - but who trained the AI? Pharma. The same companies that paid for the original studies. 🕵️♀️ This isn’t safety. It’s surveillance capitalism with a white coat.
soorya Raju
December 12, 2025 AT 10:43 AM
Bro u think this is about safety? Nah. It’s about control. The FDA doesn’t care if u live or die. They care if u follow the script. If u take a drug not on their approved list? They’ll make u pay. I know a guy who got denied insulin because his doc prescribed a ‘class-risk’ one. They gave him a generic that made him hallucinate. Now he’s in a psych ward. This is not medicine. This is a prison.
Irving Steinberg
December 13, 2025 AT 10:37 AM
So basically just ask your pharmacist? That’s it? 😅 I mean… that’s actually kinda smart. I always just Google it and panic. But yeah, pharmacists know this stuff cold. My guy at CVS just told me why my metformin is fine but my glipizide got flagged. Saved me a trip to the ER. Thanks, pharmacist heroes 🙌
Lydia Zhang
December 13, 2025 AT 13:59 PM
Class-wide alerts are unnecessary. Just label the drug. No need to scare people about the whole group.
Kay Lam
December 14, 2025 AT 09:05 AM
I’ve been a nurse for 22 years, and I can tell you - the biggest problem isn’t the warning, it’s the silence around it. Patients don’t know how to ask. Providers don’t have time to explain. And EHRs? They just pop up a red box that says ‘BLACK BOX WARNING’ with no context. I’ve sat with patients for 20 minutes just to explain why their lisinopril is still safe, even though their neighbor got off all ACE inhibitors after one news headline. We need patient-facing infographics. Simple. Visual. No jargon. This isn’t just clinical - it’s educational justice.
Adrian Barnes
December 16, 2025 AT 00:50 AM
The entire framework is fundamentally flawed. The FDA’s reliance on Proportional Reporting Ratio (PRR) is statistically naive. PRR is prone to confounding by indication, temporal clustering, and reporting bias. A PRR >2.0 is meaningless without multivariate adjustment. Furthermore, the notion that biological plausibility is a sufficient criterion for class-wide warnings is epistemologically indefensible. Correlation does not imply mechanism. The current paradigm is not evidence-based - it is heuristic-driven, and it endangers patients through both under- and over-warning. This requires a complete overhaul grounded in causal inference, not anecdotal aggregation.
Declan Flynn Fitness
December 16, 2025 AT 17:14 PM
Biggest tip I give my clients: if you see a warning, don’t panic - check the label. Look for ‘this agent’ vs. ‘all drugs in this class.’ If it’s vague? Call your pharmacist. They’ve got the cheat sheet. I’ve had patients cancel their blood pressure meds because of a headline. Then they ended up in the hospital. Don’t be that guy. Knowledge > fear. 💪
Grant Hurley
December 17, 2025 AT 00:23 AM
Wait so if one statin causes rhabdo, does that mean I can’t take any? 😅 I just thought they were all the same. Thanks for the clarity - I’m gonna check my med now. Also, DailyMed is kinda clunky but hey, at least it’s free. 🙏





patrick sui
December 1, 2025 AT 23:43 PM
Class-wide alerts are a double-edged sword. On one hand, they protect patients from systemic risks - like how all ACE inhibitors carry angioedema risk. On the other, they cause over-caution. I’ve seen patients refuse all beta-blockers because one had a black box for bronchospasm. 😕 The FDA’s PRR >2.0 threshold is solid, but real-world data is messy. We need better EHR integration - not just labels, but contextual pop-ups when prescribing.