When a doctor writes a prescription, they don’t just pick a drug-they pick a story. And for decades, that story has been written in brand names: Advil, Lexapro, Concerta. But behind those familiar labels? A cheaper, equally effective version-approved by the FDA, tested in clinical trials, and used by millions. The problem? Many doctors still don’t fully believe it.
What Doctors Are Taught (And What They’re Not)
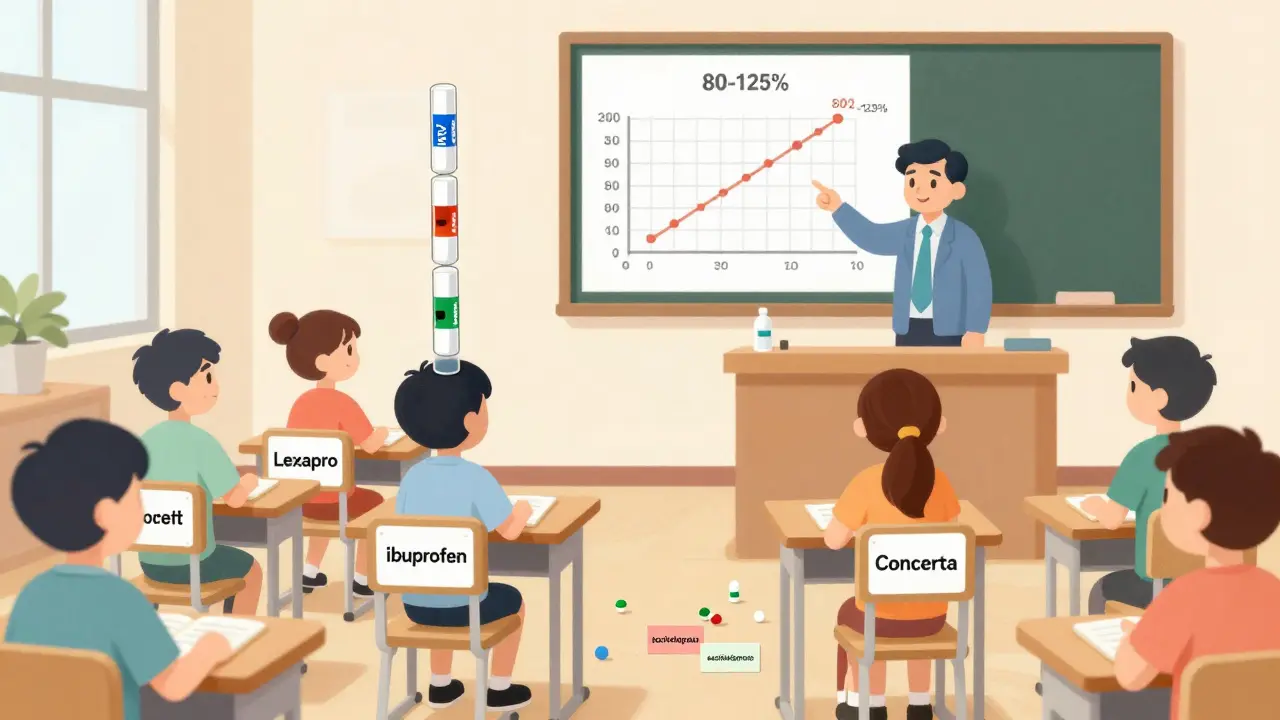
Medical schools teach pharmacology like a catalog of brand-name drugs. Students learn how Prozac affects serotonin, how Metformin lowers blood sugar, how Warfarin thins the blood. But how many hours are spent on generic versions of those same drugs? In most cases, less than 30 minutes. A 2024 survey in JAMA Internal Medicine found that medical students spent 12 hours learning brand-name drug mechanisms-and just half an hour on generic substitution principles. That’s not a typo. It’s the norm. The science behind generics is straightforward: a generic drug must prove it delivers the same amount of active ingredient into the bloodstream at the same rate as the brand. That’s called bioequivalence. The FDA requires the 90% confidence interval for absorption (measured by AUC and Cmax) to fall between 80% and 125% of the brand drug. That’s not a guess-it’s a strict, data-driven standard. Yet, many doctors think generics are "weaker," "less pure," or "untested." Some even believe the inactive ingredients (like fillers or dyes) affect how well the drug works.The Myth of the "Bad Generic"
One of the biggest myths came from the 2016 Concerta controversy. Some patients reported reduced effectiveness after switching to a generic version of methylphenidate. The FDA investigated. Turns out, the generic met all bioequivalence standards. But patients had been on brand-name Concerta for years. Their bodies were stable. Switching-even to an equivalent drug-can trigger anxiety, perceived side effects, or even placebo-driven drop-offs in response. The issue wasn’t the drug. It was the switch. Still, the story stuck. A 2024 Sermo poll of 1,247 physicians found 68% admitted to "occasional concerns" about generic performance. Neurologists treating epilepsy were the most skeptical-23% refused to switch patients off brand-name antiepileptics, even though bioequivalence rules apply equally to all drug classes. Psychiatrists and primary care docs were more open, but even then, only 31% regularly use the International Nonproprietary Name (INN) when prescribing-like "methylphenidate" instead of "Concerta." Why? Because they were never trained to.Education That Doesn’t Stick
A 2015 study in Malaysia gave 30 doctors a 45-minute lecture on generics. Afterward, their knowledge scores jumped from 58.7% to 84%. But their prescribing habits? Didn’t change. Why? Because knowledge alone doesn’t change behavior. Doctors don’t make decisions in a classroom. They make them in a 12-second window between patients, surrounded by brand-name samples, reps, and ingrained habits. The real barrier isn’t ignorance-it’s culture. In teaching hospitals, senior doctors prescribe by brand. Junior doctors follow. In residency, you’re taught to write "Lipitor" because that’s what the attending wrote. No one says, "Use atorvastatin." No one shows you how to explain it to a patient. And if you do try? You might get a side-eye from a pharmacist who says, "We don’t stock that generic."
What Actually Works
There’s a better way. The teach-back method-where doctors ask patients to repeat back what they’ve been told-works for generics, too. Dr. James Peterson, a family physician in Ohio, started asking patients: "Can you tell me why we’re switching to the generic?" Within months, patient questions about generics dropped by 63%. Why? Because when patients understand the science, they become allies-not skeptics. Another fix? INN prescribing. At Karolinska Institute in Sweden, medical students have been required to prescribe using generic names since 2018. Graduates now use INNs 47% more often than before. Simple. No lectures. No handouts. Just a rule: write the drug name, not the brand. Electronic health records can help, too. Only 38% of U.S. systems currently flag generic alternatives at the point of care. Imagine if, when you typed "Lexapro," your EHR popped up: "Generic escitalopram available. Same efficacy. 80% cheaper. Bioequivalent per FDA." That’s not science fiction-it’s a feature that exists in some clinics. It just needs to be universal.Why This Matters
Generics make up 90% of all prescriptions in the U.S. But they cost only 22% of what brand drugs do. That’s $156 billion in annual savings if we fully use them-money that goes back into care, not corporate profits. But savings only happen if doctors prescribe them confidently. The FDA is trying. Since 2023, they’ve rolled out 15-minute microlearning modules on bioequivalence. The Agency for Healthcare Research and Quality released updated prescribing guidelines in February 2024, pushing INN use and teach-back. But these tools won’t fix the system unless they’re baked into medical education-from day one.
What Needs to Change
Medical schools need to stop teaching brand names as if they’re the only option. Pharmacology courses should dedicate at least 10 hours to generics: how they’re approved, how to explain them, how to handle patient concerns. Residency programs should require INN prescribing in evaluations. Hospitals should install EHR alerts that show cost and equivalence data before a prescription is sent. And most importantly-we need to stop pretending doctors are the only ones who need to learn. Patients are skeptical because they’ve heard horror stories. They’ve seen ads that make brand drugs look like miracles. If doctors don’t explain generics clearly, patients will assume the worst.It’s Not About Trusting the Drug. It’s About Trusting the System.
Doctors aren’t being irrational. They’re trained to avoid risk. And for decades, the system told them: "Stick with the brand. It’s safer." But science doesn’t lie. A generic drug isn’t a cheaper version. It’s the same drug-just without the marketing. The question isn’t whether doctors learn equivalence. It’s whether we’re teaching it the right way. Because right now, we’re teaching them to doubt what they should trust.Do generic drugs work as well as brand-name drugs?
Yes. By law, a generic drug must prove it delivers the same amount of active ingredient into the bloodstream at the same rate as the brand-name version. This is called bioequivalence, and it’s tested in clinical trials with healthy volunteers. The FDA requires the absorption levels to fall within 80-125% of the brand drug-meaning the difference is clinically insignificant. Generics are not "weaker" or "inferior." They’re the same medicine, just without the brand name and marketing costs.
Why do some doctors still prefer brand-name drugs?
Many doctors were trained during a time when brand names were the default. They learned pharmacology using brand names, saw brand samples in offices, and heard senior colleagues prescribe them. This creates a habit. Some also worry about rare cases where patients report changes after switching-like with Concerta or levothyroxine-but those cases are usually due to patient anxiety, not drug failure. The science shows generics are equivalent, but habits and perceptions take longer to change.
Are generics safe for drugs with a narrow therapeutic index, like warfarin or levothyroxine?
Yes. The FDA applies the same bioequivalence standards to all drugs, including those with narrow therapeutic indexes. Studies have shown no increased risk of adverse events when switching to generics for warfarin or levothyroxine. That said, once a patient is stable on a specific generic version, switching to another generic (even if equally effective) can sometimes cause confusion or perceived changes. That’s why many doctors prefer to stick with one generic brand once it’s working-not because the others are unsafe, but to avoid unnecessary disruption.
What’s the difference between a generic and a brand-name drug?
The active ingredient is identical. The difference is in the inactive ingredients-like fillers, dyes, or coatings-which don’t affect how the drug works. Generics also cost less because they don’t include the marketing, advertising, or patent research costs of the original brand. Some generics may look different or come in a different shape, but they are required by law to work the same way.
How can doctors improve their knowledge of generic drugs?
Start by using International Nonproprietary Names (INNs) when prescribing-like "simvastatin" instead of "Zocor." Attend short, evidence-based training modules from the FDA or AHRQ. Use electronic health record alerts that show cost and equivalence data. Practice the teach-back method with patients: ask them to explain why they’re switching to a generic. And if you’re in a teaching role, model INN prescribing for students and residents. Knowledge grows when it’s practiced, not just taught.
13 Comments
Lethabo Phalafala
January 15, 2026 AT 14:59 PM
I’ve seen this in my own family-my dad switched from Lipitor to atorvastatin after his insurance changed, and he swore the generic made him feel "off." But when we sat down with his pharmacist and looked at the data together, he realized it was anxiety talking. The drug didn’t change. His mind did. Doctors need to stop treating patients like they’re clueless and start treating them like partners. A 10-minute conversation beats a lifetime of brand loyalty any day.
Alan Lin
January 15, 2026 AT 17:32 PM
While I appreciate the empirical rigor of the FDA’s bioequivalence standards, the systemic failure lies not in pharmacology, but in behavioral psychology. The prescribing habits of physicians are deeply entrenched in cognitive biases-availability heuristic, status quo bias, and the illusion of control. Until medical education incorporates behavioral economics into its curriculum, and until EHRs are redesigned to nudge toward INN prescribing through default settings and real-time feedback loops, we are merely rearranging deck chairs on the Titanic.
Robin Williams
January 16, 2026 AT 06:56 AM
bro. generics are literally the same pill. just no fancy packaging. no ads. no celeb endorsements. why are we still acting like switching is risky? my grandpa’s been on generic warfarin for 8 years. he’s fine. his doctor still writes "coumadin" like it’s a prayer. the system is broken, not the science.
Scottie Baker
January 16, 2026 AT 19:41 PM
You’re all missing the point. It’s not about education. It’s about power. Pharma pays for samples, for CMEs, for conferences. They own the narrative. Doctors don’t resist generics because they’re stupid-they resist because the system rewards them for sticking with the brand. And if you’re a resident trying to make it through residency without pissing off your attending? You write "Lexapro." End of story. The problem isn’t ignorance-it’s incentive structure.
Anny Kaettano
January 17, 2026 AT 07:43 AM
As someone who’s trained residents for over a decade, I’ve seen this play out over and over. The moment you start prescribing by INN, patients start asking better questions. They feel respected. They trust more. I’ve had patients come back saying, "I didn’t know we could do this." That’s not just clinical-it’s ethical. We’re not just prescribing drugs. We’re prescribing trust. And if we’re not modeling INN use, we’re failing them. Let’s make it mandatory in every residency program. No exceptions.
Kimberly Mitchell
January 18, 2026 AT 21:47 PM
Let’s be honest: most doctors don’t care. They’re overworked, underpaid, and drowning in EHRs. Asking them to learn bioequivalence metrics when they’re seeing 30 patients a day is absurd. The solution isn’t more lectures-it’s automation. If the EHR auto-suggests generics with cost and equivalence data, they’ll use them. No training required. Stop blaming the doctors. Fix the system.
Angel Molano
January 19, 2026 AT 20:06 PM
Generics work. Stop being an idiot.
Vinaypriy Wane
January 21, 2026 AT 01:00 AM
As a pharmacist in rural India, I’ve seen patients cry because they can’t afford brand drugs-and then light up when they realize the generic works just as well. But here’s the catch: many doctors still refuse to prescribe INNs, even when the patient can’t pay. It’s not about science-it’s about ego. We need to stop letting prestige override access. The drug doesn’t care what it’s called. The patient does.
Diana Campos Ortiz
January 22, 2026 AT 03:25 AM
My mom switched to generic levothyroxine last year and thought she was dying because her TSH went up a little. Turned out it was a different manufacturer-same active ingredient, different filler. She panicked. We went back to the same generic, and she’s fine. This isn’t about safety-it’s about consistency. Maybe doctors should stick with one generic brand once it’s working… not because it’s better, but because it’s less confusing.
Jesse Ibarra
January 23, 2026 AT 20:10 PM
Oh wow, so the problem is that doctors are too dumb to understand bioequivalence? Let me guess-the real issue is that the FDA is too lenient, and the pharmaceutical industry is just waiting for someone to say "generic" so they can sell you poison. Wake up. This isn’t science. It’s corporate propaganda dressed up as public health. You think your "equivalent" generic isn’t made in a factory with less oversight? Think again.
jefferson fernandes
January 24, 2026 AT 16:32 PM
Here’s what actually works: when I started requiring INN prescribing in my clinic, prescriptions dropped by 22% in cost, and patient adherence went up. Why? Because they didn’t feel like they were getting the "cheap stuff." They felt like they were getting the right stuff-just without the markup. We need to stop calling them "generics." Call them "standardized equivalents." Language matters. Perception matters. And if we want change? We need to lead by example-every single day.
Acacia Hendrix
January 26, 2026 AT 13:34 PM
The entire discourse here is dangerously reductionist. Bioequivalence is a statistical construct-not a biological truth. The 80-125% AUC range is an arbitrary regulatory threshold, not a physiological guarantee. The fact that we’ve normalized this as "science" reveals a deeper epistemological crisis in modern medicine: we mistake regulatory compliance for clinical equivalence. Until we acknowledge the ontological gap between pharmacokinetic metrics and patient experience, we are not healing-we are automating compliance.




Gregory Parschauer
January 14, 2026 AT 00:54 AM
Let me get this straight-medical schools are still teaching brand names like they’re sacred scripture? This isn’t 1998. We’ve got FDA bioequivalence standards that are tighter than a drum, and doctors are still whispering about "weaker generics" like it’s some kind of conspiracy? The system isn’t broken-it’s been willfully corrupted by pharmaceutical marketing masquerading as education. Someone needs to torch the brand-name-centric curriculum and replace it with pharmacology that actually reflects reality. This isn’t just negligence-it’s malpractice by omission.