FDA Warning: What It Means and Which Medications Are at Risk
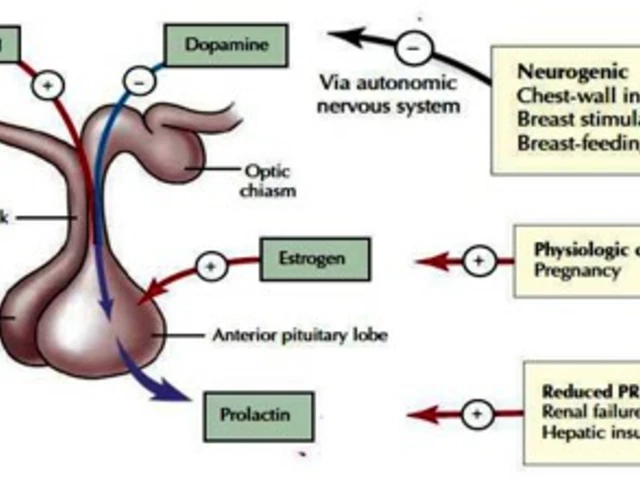
When you see an FDA warning, a public alert issued by the U.S. Food and Drug Administration about a medication’s serious safety risk. Also known as a black box warning, it’s not a suggestion—it’s a red flag that a drug can cause life-threatening harm if used carelessly. These warnings don’t come lightly. They’re based on real cases of organ failure, sudden death, or permanent injury linked to specific medicines. You’ll find them on labels for drugs like fluoroquinolone antibiotics, which can tear tendons without warning, or anticholinergics that quietly raise dementia risk over years of use.
The FDA REMS, a safety program that forces pharmacies and doctors to follow strict rules for high-risk drugs, often goes hand-in-hand with these warnings. For example, if a drug can cause a deadly skin reaction like DRESS syndrome, the FDA doesn’t just slap on a label—it requires special training, patient registries, and even limits on who can prescribe it. Then there’s the medication recall, when a drug is pulled from shelves because it’s contaminated, mislabeled, or unexpectedly dangerous. These aren’t rare. In 2023 alone, over 300 drugs were recalled in the U.S., many tied to hidden interactions or faulty manufacturing. And while recalls grab headlines, the quiet, slow-burning dangers—like how smoking cuts the effectiveness of your meds, or how expired OTC pills lose potency—are just as real.
These aren’t abstract risks. They show up in your medicine cabinet. That aspirin you take daily? It can turn deadly if mixed with blood thinners. Your antifungal pill might not work because your stomach acid reducer blocks it. Even your sleep aid could be linked to memory loss over time. The FDA warning exists because these aren’t hypotheticals—they’re documented, preventable tragedies. The posts below cover exactly these scenarios: the drugs that cause tendon ruptures, the combinations that trigger internal bleeding, the ingredients in generics that cause hidden reactions, and the forgotten warnings buried in fine print. You’ll find what to watch for, what to ask your doctor, and how to act before it’s too late.
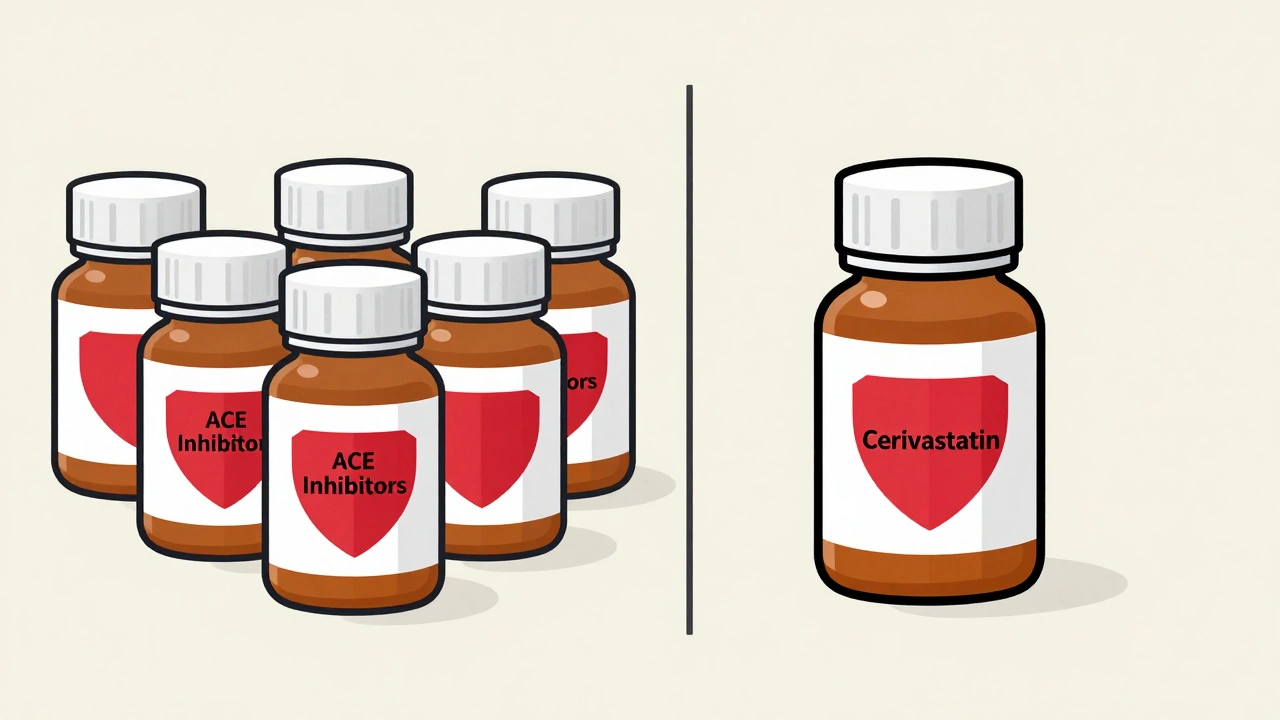
How to Identify Class-Wide vs. Drug-Specific Safety Alerts in Medications
Learn how to tell the difference between class-wide and drug-specific safety alerts from the FDA. Understand why some warnings apply to all drugs in a group and others only to one-and how to make safer decisions.
View More